Molecular interactions occur between molecules or atoms that are not covalently bonded to one another.
They are relevant to biology as they form the framework of all life processes.
They contribute to the formation of cellular structures, the storage, and expression of genetic information, metabolic activities, and the execution of complex cellular communication.
Unit-1 of the CSIR NET/JRF Life Sciences Examination is Molecules and Their Interaction Relevant to Biology.
Check updated information on CSIR UGC NET – Life Sciences
Structure of Atoms, Molecules, and Chemical Bonds
This section introduces the structure of atoms, molecules, and chemical bonds. It is part of the life science syllabus to make us understand the structure of the atom.
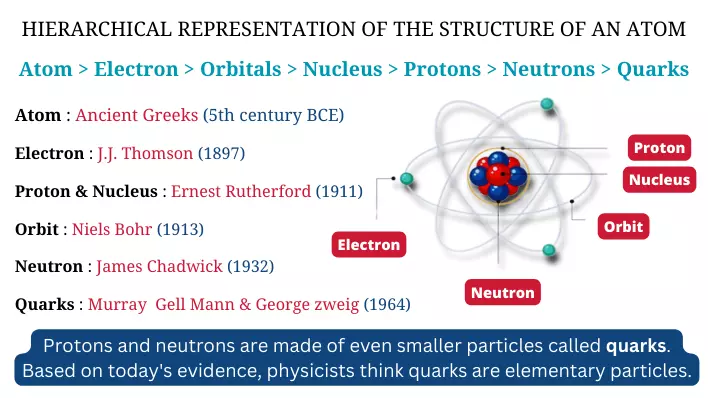
Some important terms that make the base for this unit are atom, molecule, element, compound, and mixture:
An atom is the smallest unit of an element that maintains the properties of that element. It is identified primarily by its atomic number, corresponding to the number of protons in its nucleus.
An element is a pure substance composed of only one type of atom, e.g., carbon (C) and oxygen (O).
A molecule is formed when two or more atoms join with a chemical bond. These joined atoms can be the same as in O2 (Oxygen molecule) or different as in H2O (Water molecule).
A compound is a molecule that contains at least two different types of elements.
“All compounds are molecules, but not all molecules are compounds” (true Covalent Compounds, not true for ionic compounds)
Finally, a mixture combines two or more substances that retain their chemical identity but are physically mixed together, preserving their properties and allowing them to be physically separated.
How studying atomic structure is important for biological research?
The atomic structure has a significant role in structural biology; researchers use methods like X-ray crystallography and Nuclear magnetic resonance (NMR) to map out the precise position and the movements of every atom within a biomolecule to find its 3D structure.
Now we have already seen what molecules are, let’s move to the next section, “Composition, structure, and function of biomolecules,” to explore everything about biomolecules.
Composition, structure, and function of biomolecules
The previous section covers a molecule, a group of two or more atoms held together by chemical bonds.
What are biomolecules?
A biomolecule or biological molecule is a specific type of molecule present in living organisms.
Biomolecules are a subset of molecules that play an important role in biological processes due to their specific structures and functions. However, not all molecules can be classified as biomolecules.
These biomolecules include large molecules like proteins, nucleic acids, carbohydrates, lipids, vitamins, and some small molecules, such as primary and secondary metabolites.
But this section covers main biomolecules like carbohydrates, proteins, nucleic acids, lipids, and vitamins.
The function of biomolecules mentions above are:
- Carbohydrates are the main source of energy
- Lipids form the cell membrane
- Proteins provide structural support
- Vitamin assist various metabolic processes
- Nucleic acid stores the genetic information.
So, the above two sections explain the structure of the atom and the formation of biomolecules, and the function of biomolecules.
In metabolic processes, the biomolecules interact; the point of interest is how these interactions occur and what makes them stable.
The third section is dedicated to stabilizing interactions like hydrogen bonding, van der Walls, electrostatic, and hydrophobic interaction
Stabilizing interaction
Stabilizing interactions, also called noncovalent interactions, are crucial in maintaining the structure and function of biomolecules such as proteins, nucleic acids, and lipids. These interactions are weaker than covalent bonds but are nonetheless critical because of their impact on biomolecular interactions’ molecular shape, stability, and specificity.
Importance of stabilizing interactions:
- Structure and function of molecules: stabilizing interactions such as van der Waals forces, electrostatic interactions, hydrogen bonding, and hydrophobic interactions determine the three-dimensional structure of biomolecules.
- Protein Folding and Stability: Stabilizing interactions are crucial for protein folding because they determine the stable conformation of a protein.
- Enzyme-substrate interactions: Stabilizing interactions between enzymes and their substrates plays a crucial role in enzyme catalysis.
Examples of Stabilizing interactions :
- Van der Waals Forces: These are weak, short-range electrostatic attractive forces between uncharged molecules that arise from the interaction of permanent or transient electric dipole moments. They are important for maintaining the tertiary structure of proteins and the double helix structure of DNA.
- Hydrogen Bonds: This type of bond is formed when a hydrogen atom is shared by two other atoms, usually oxygen or nitrogen. Hydrogen bonds are critical to the structure of proteins and nucleic acids as they help stabilize their three-dimensional structures.
- Hydrophobic Interactions occur between non-polar groups or molecules in an aqueous (water-based) environment. The non-polar groups tend to cluster together to minimize contact with water. This interaction is critical for maintaining the folded structure of proteins and the integrity of biological membranes.
- Electrostatic Interactions occur between permanently charged or ionizable groups and play a crucial role in biomolecular structure and function. They are stronger than Van der Waals forces and can operate longer distances.
- Ionic Interactions are electrostatic attractions between charged atoms or groups, often between positively charged (cationic) and negatively charged (anionic) groups. Many biological structures, including proteins and DNA, contain ionic interactions.
- Pi-Pi Stacking: This is an attractive, noncovalent interaction between aromatic rings, such as those found in nucleobases. It plays an important role in stabilizing the structure of DNA and RNA.
- Cation-Pi Interaction: A non-covalent molecular interaction between a cation’s positive charge and the electron cloud’s negative charge in an aromatic system. This interaction plays a key role in stabilizing protein structures and protein-ligand interactions.
- Dipole-Dipole Interactions: These interactions occur between the positive end of one polar molecule and the negative end of another. They are weaker than hydrogen bonds but still contribute to the stability of biomolecular structures.
- Metal Ion Coordination: Some biomolecules interact with metal ions to stabilize their structure. For example, zinc fingers are structural motifs stabilized by zinc ions in certain DNA-binding proteins.
These interactions, individually or in combination, contribute to various biological systems’ structural integrity and functionality.
Principles of biophysical chemistry
Biophysical chemistry is an interdisciplinary field that applies the theories and methods of physics and chemistry to understand biological systems. The principles of biophysical chemistry represent the fundamental theories and concepts derived from physics and chemistry and applied to understanding and explaining biological phenomena.
Biophysical chemistry uses these principles to study the physical properties of biological molecules, how they interact, and how they create life’s complexity.
Fundamental Principles of biophysical chemistry
- pH: pH measures the concentration of hydrogen ions in a solution and indicates its acidity or alkalinity. It is crucial for many biological processes since biological molecules’ structure and function often depend on pH. The pH scale ranges from 0 (very acidic) to 14 (very alkaline), with seven being neutral.
- Buffers: Buffers play an important role in biological systems by resisting changes in pH when small amounts of acid or base are added. These solutions help keep the pH value in a narrow, life-compatible range. An example of this is the bicarbonate buffer system present in the blood, which maintains its pH at around 7.4.
- Reaction Kinetics: The study of reaction kinetics involves studying the rates at which biochemical reactions occur, how temperature and concentration affect those rates, and the mechanisms by which the reactions occur. For example, enzyme kinetics examines the speed of enzyme-catalyzed reactions and their regulation.
- Thermodynamics: This is the study of energy and its transformations. In biological systems, thermodynamics helps explain processes such as ATP (adenosine triphosphate) synthesis, enzyme catalysis, and protein folding. Key concepts include enthalpy (the heat content of a system), entropy (a measure of disorder), and Gibbs’s free energy (which predicts the spontaneity of a reaction).
- Quantum Mechanics and Spectroscopy: The principles of quantum mechanics allow us to understand the behavior of molecules at the atomic level, which is crucial for elucidating biological processes. Scientists often use spectroscopy to identify and study molecules and their interactions based on the principles of quantum mechanics.
- Statistical mechanics: Statistical mechanics explains the macroscopic properties of systems in terms of their microscopic components. For example, it allows an understanding of how changes in the conformation of a protein molecule can affect its macroscopic properties.
- Electrochemistry: This is the study of chemical reactions involving electron transfer. In biology, it is crucial to understand processes such as nerve transmission, photosynthesis, and cellular respiration.
- Biophysical Techniques: Researchers apply principles of biophysical chemistry in the development and use of various analytical techniques, including X-ray crystallography, nuclear magnetic resonance (NMR), electron microscopy, and various types of spectroscopy to study biological molecules.
Bioenergetics and Dynamics
Biological systems are powered by complex biochemical reactions and processes that allow life to thrive. Understanding these processes is crucial to unraveling the mechanisms behind biological functions and diseases.
Bioenergetics: This is the study of energy conversion in living organisms. It includes the study of photosynthesis in plants and energy production through processes such as glycolysis and oxidative phosphorylation in animals.
Glycolysis is the process in cell metabolism where a glucose molecule is broken down into two pyruvate molecules, releasing the energy stored in the glucose molecule. Energy is captured in ATP (adenosine triphosphate), the main energy currency in cells, and NADH, which is used in other biochemical reactions.
Oxidative phosphorylation is the metabolic pathway in which cells use enzymes to oxidize nutrients, releasing the energy needed to produce ATP. It is the last stage of cellular respiration that occurs in the mitochondria and produces most of the ATP in eukaryotic cells.
Coupled Reactions: In biochemistry, reactions often occur in pairs, with one reaction driving the other. The energy-releasing exergonic reaction provides the energy needed for the energy-consuming endergonic reaction. An example is the coupling of ATP hydrolysis (exergonic) to drive reactions such as the synthesis of macromolecules (endergonic).
Group Transfer: This is the transfer of a functional group from one molecule to another. For example, the transfer of a phosphate group from ATP to another molecule during phosphorylation. This is an important part of signal transduction pathways and metabolism.
Biological Energy Transducers: These are systems or components of cells that convert energy from one form to another. Examples include ATP synthase (which converts the driving force of protons into chemical energy in the form of ATP), photosystems in photosynthesis (which convert light energy into chemical energy), and mitochondria (which convert chemical energy from food into usable energy for the cell ).
Principles of catalysis, enzymes, and enzyme kinetics
Enzymes play a crucial role in biological systems because they catalyze (accelerate) chemical reactions necessary for life. They exhibit several important properties that make them incredibly efficient and specific catalysts.
Principles of Catalysis
Catalysts increase the rate of a chemical reaction by lowering the energy barrier, or activation energy, required for the reaction to occur. They achieve this by providing an alternative reaction pathway with lower activation energy. Catalysts are not consumed in the reaction, so they can function repeatedly.
Enzymes
Enzymes are biological catalysts, typically proteins, that speed up biochemical reactions. They have exceptional catalytic power and specificity. Enzymes bind their substrates to a specific site, the active site, where the chemical reaction occurs.
Enzyme Kinetics
This is the study of the rates at which enzymes catalyze reactions. The rate at which an enzyme works can be affected by substrate concentration, enzyme concentration, temperature, pH, and the presence of inhibitors or activators.
Enzyme Regulation
The activity of enzymes can be regulated in several ways to ensure they only work when needed. Regulatory mechanisms include allosteric regulation (where a molecule binds to an enzyme at a site other than the active site and alters its activity), covalent modification (such as phosphorylation), and the control of enzyme synthesis and enzyme degradation.
Mechanism of Enzyme Catalysis
Enzymes catalyze reactions by stabilizing the transition state and reducing the activation energy required for the reaction. They do this by binding substrates in a specific orientation, creating a favorable microenvironment, or participating directly in the reaction.
Isozymes (or isoenzymes)
Isozymes, also known as isoenzymes, catalyze the same reaction, but different genes encode them, and they may have different properties, such as optimal temperatures or pH levels, locations in the body, or regulation. They represent a backup system for the body, wherein one enzyme can compensate if another fails or gets inhibited.
Conformation of proteins
Proteins are complex biomolecules whose function depends crucially on their three-dimensional structure.
Ramachandran Plot: Developed by G.N. Ramachandran, a Ramachandran plot is a graphical representation of the dihedral (torsional) angles – psi (ψ) and phi (φ) – of a protein’s backbone. These angles are crucial for understanding the conformation of the protein. Certain combinations of ψ and φ angles correspond to specific secondary structures like alpha helices and beta sheets.
Secondary Structure: This refers to the local spatial arrangement of the protein’s backbone, typically represented by alpha (α) helices and beta (β) sheets. Hydrogen bonds stabilize alpha helices as right-handed coils, while beta sheets form a pleated sheet by connecting beta strands laterally through at least two or three backbone hydrogen bonds.
Domains: Domains are different functional and/or structural units in a protein. They are responsible for specific functions and can often fold and function independently of the rest of the protein chain. A single protein can have multiple domains.
Motif (or super-secondary structure): A motif is a specific combination of several secondary structural elements, which often have a specific function. Motifs are smaller than domains and often serve as building blocks of domains. Common examples are the helix-loop-helix and zinc finger motifs.
Folds: A protein fold refers to the general architectural theme of protein structure. Many proteins with different sequences can share the same fold if their structures are similar. Classifying protein structures into folds helps us understand the general principles of protein folding and function.
Studying protein conformation is critical to understanding how proteins perform their specific functions within the cell. It is also fundamental in drug design, where the goal is often to design small molecules that can specifically interact with a protein of interest based on the structure of the protein.
Conformation of nucleic acids
Nucleic acids, including DNA and RNA, are vital biomolecules with genetic information. Similar to proteins, the conformation of nucleic acids determines their function. Here is a brief description of the Conformation of nucleic acids (helix (A, B, Z), t-RNA, micro-RNA).
- Helix (A, B, Z): These represent different forms of the DNA double helix.
- Under physiological conditions, the most common form of DNA is the B form, which textbooks commonly present. It is a right-handed helix with approximately 10.5 base pairs per turn is a right-handed helix with about 10.5 base pairs per turn.
- Like the B form, the A form of DNA is a right-handed helix but shorter and broader. This form is less common in vivo but can occur under dehydrating conditions or in hybrid DNA-RNA structures.
- The Z form of DNA is a left-handed helix, which differs significantly from the A and B forms. It is less common but could play a role in gene regulation.
- t-RNA: Transfer RNA (tRNA) is a type of RNA that helps decode a messenger RNA (mRNA) sequence into a protein. tRNA has a complex 3D structure, often represented as a cloverleaf in the flat state and as an L-shape in 3D. It has an anticodon region that pairs with the mRNA codon and an amino acid binding site at the opposite end.
- Micro-RNA (miRNA): MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression post-transcriptionally. They bind to the 3′ untranslated regions of target mRNAs, degrade them, or inhibit translation. Proteins in the miRNA processing machinery recognize the short hairpin structures of miRNAs, although their 3D structures can vary.
Understanding the conformation of nucleic acids is crucial for many biological and medical applications, including gene therapy, disease diagnosis, and new drug development.
Stability of proteins and nucleic acids
The stability of proteins and nucleic acids, essential for their function, is based on a delicate balance of various intramolecular and intermolecular forces.
Factor affecting the protein stability:
- Non-Covalent Interactions: Proteins fold into a specific three-dimensional structure driven by non-covalent interactions such as hydrogen bonding, ionic interactions, van der Waals forces, and hydrophobic interactions.
- Disulfide Bonds: These covalent bonds between two cysteine residues in a protein contribute significantly to protein stability, particularly in extracellular proteins.
- pH and Ionic Strength: The pH and ionic strength of a protein’s environment can affect the protein’s charge distribution, alter the balance of attractive and repulsive forces, and affect protein stability.
- Temperature: High temperatures can disrupt the weak noncovalent interactions holding a protein together, leading to denaturation.
- Post-Translational Modifications: Modifications such as phosphorylation, methylation, and glycosylation can affect the stability of a protein.
Factor affecting the nucleic acid stability:
- Hydrogen Bonds: In DNA and RNA, base pairs are held together by hydrogen bonds-two for A-T (or A-U) pairs in RNA and three for G-C pairs. The more G-C pairs a molecule has, the more stable it is due to the extra hydrogen bonding.
- Base Stacking Interactions: Hydrophobic base stacking interactions within the helix also enhance nucleic acid stability.
- Ionic Strength and pH: High concentrations of positive ions can stabilize the negatively charged backbone of nucleic acids. Likewise, pH can affect stability since changing pH can alter the protonation state of bases and disrupt base pairing.
- Temperature: High temperatures can disrupt hydrogen bonds and base stacking interactions, leading to denaturation (melting) of the DNA or RNA molecule.
- DNA methylation: Methylation of cytosine bases in DNA (formation of 5-methylcytosine) can also affect the stability of the DNA structure.
Understanding the factors contributing to protein and nucleic acid stability is crucial in drug design, genetic engineering, and diseases associated with protein misfolding, such as Alzheimer’s and Parkinson’s.
Metabolism of Biochemical Building Blocks
Metabolism sustains life and facilitates growth, reproduction, and response to the environment. It involves complex chemical reactions and can be classified into catabolism and anabolism.
- Catabolism breaks down organic matter through cellular respiration for energy.
- Anabolism uses energy to build cellular components such as proteins and nucleic acids.
Carbohydrate Metabolism: Carbohydrate metabolism involves the primary breakdown of carbohydrates by glycolysis, producing pyruvate. Pyruvate then enters the citric acid cycle (known as the Krebs cycle or TCA cycle), where further breakdown occurs, releasing energy and reducing the energy stored in ATP as NADH and FADH2. Carbohydrate metabolism also includes gluconeogenesis, which synthesizes glucose from non-carbohydrate precursors.
Lipid Metabolism: Lipid metabolism primarily involves the oxidation of lipids and the breakdown of fatty acids to generate acetyl-CoA. This molecule can then enter the citric acid cycle. In addition, a process called lipogenesis synthesizes lipids from acetyl-CoA.
Amino Acid Metabolism: Amino acids can be both synthesized and broken down. Amino acid synthesis is the formation of new amino acids from core molecules, while degradation often breaks down amino acids into components that can enter the citric acid cycle.
Nucleotide Metabolism: Nucleotides, the DNA and RNA building blocks, can also be synthesized and broken down. Nucleotide synthesis can be either de novo (from new, using simple molecules as substrates) or salvage (recycling free bases and nucleosides released upon nucleotide degradation). The breakdown of nucleotides ultimately leads to the formation of uric acid or urea, which is excreted from the body.
Vitamin Metabolism: The body digests, absorbs, and converts vitamins into active forms in vitamin metabolism. Sourced primarily from food because the body cannot produce them, these essential nutrients are small organic compounds that significantly support overall health. For example, sunlight directly contacting the skin triggers vitamin D synthesis from cholesterol. The liver and kidneys then activate this synthesized vitamin D.